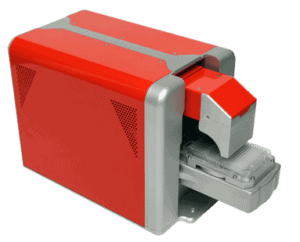
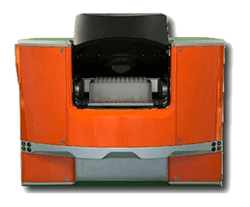
INSTRUMENTS DESIGNED FOR YOUR NEEDS
Aura™ family systems help you develop stable, pure, and efficacious protein, cell, and gene therapies faster. They combine Fluorescence Membrane Microscopy (FMM) technology and Backgrounded Membrane Imaging (BMI) technology to give you high-throughput particle count, size, and ID information at the volume that fits best with your workflow. Plus, you won’t have to worry about missing translucent particles not seen with liquid-based methods.

From as low as 5 µL, get rapid, accurate and quantitative particle distribution in your sample. Fluorescence detection offers application versatility, ensuring that all your aggregate and particle analysis needs are met.
Aura systems determine drug product quality using BMI, a fluidics-free technology with no risk of system clogging. BMI’s refractive index contrast is 10 x greater than with liquid based measurements, providing significantly increased particulate detection sensitivity.
Meets USP <788> and <1788> recommendations – automation-ready, simple and robust workflow, and able to handle complex, high-viscosity, and low-volume samples.
Aura’s 96-well automated format is highly suited to DoE, with analysis taking about 1 minute per sample, and no dilution or sample preparation required.

Technology
BMI uses sophisticated image processing techniques to analyse images and acquire particle data. The key is to first take a background image of the membrane. After samples are filtered through and particles are captured, the same membrane is re-imaged, this time with particles on the surface. The “background image” is precisely aligned with the “measure image” and then subtracted on a pixel by pixel basis so that the background texture is eliminated and particles are revealed.
Refractive index contrast in BMI is 10x greater than measurements done in liquid, such as light obscuration and flow imaging. BMI offers the increased sensitivity required to conduct robust early stage particulate detection. Particle sizes are calibrated with an electron microscope and analysis is fully automated.
Example image analysis identifying protein in subvisible aggregate particles
(a) Brightfield (b) Darkfield (c) Fluorescence (d) Combined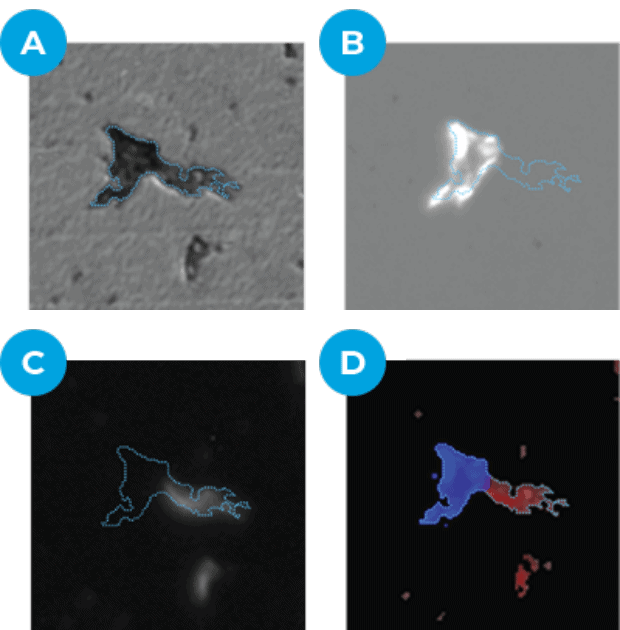
Example image analysis identifying protein in subvisible aggregate particles
(a) Brightfield (b) Darkfield (c) Fluorescence (d) Combined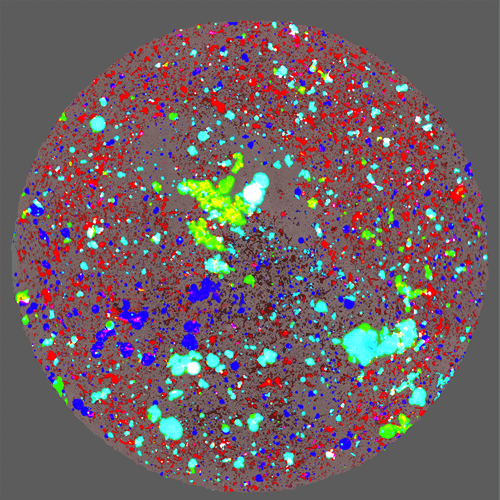
FMM works with BMI on the Aura to tell you exactly what is in your formulation. It utilises fluorescent reagents that bind specifically to a particular particle population for simple and definitive identification that doesn’t depend on potentially misleading morphology and intensity data.
The additional utility of fluorescence enables you to distinguish protein from non-protein aggregates, capsids and cells from aggregates, track DNA leakage and polysorbate degradation.


APPLICATIONS
Injectable formulation development and quality control
According to the FDA, drug manufacturers can’t just rely on the minimum standards when they release a product. They need to make sure that their products are truly safe and so particles need to be fully characterised throughout the entire development cycle. Halo Labs allow drug companies to make critical therapeutics safer and reduce their development time to help patients in need.
Halo’s Aura line of instruments are the first systems that allow particles to be tested both early in development and later during quality control. The Aura systems can determine if a particle is the result of the drug itself, an ingredient in the liquid crashing out of solution, an external contaminant, or even a cell.
Therapeutic areas:
- Biologics
- Gene Therapies
- Cell Therapies
- Small Molecules
Biologics
From developability assessment to lot release
Aura systems generate USP <788> compliant subvisible particle data for lot release. Aura systems are the industry’s first analytical system designed specifically to address the demand for quantitative subvisible particle analysis in high-throughput, even when limited sample material is available.
Aura systems monitor product quality and stability as early as candidate selection, during development of higher concentration formulations and through to product release. With consistent counts from low to high sample volumes, Aura PTx is the ideal system for final product testing to confirm product quality in many of the biopharmaceutical products currently in development.
Aura’s 96-well automated format is highly suited to DoE approach to formulations development. Rapid analysis means that several parameters can be tested in a short amount of time. Perform an excipient screen, pH screen, or test buffer conditions in a matter of hours. Aura PTx’s fluorescence applications allow you to ID protein from non-protein aggregates and track polysorbate degradation, to determine biologic and polysorbate stability.
Assess for developability
Assess degraded excipients
Lot-Release SVP testing
Gene Therapies
Ultra-low volume AAV aggregate quantitation and ID
As parenterally administered drugs, gene therapies must undergo the same rigorous safety testing required by the FDA for protein therapeutics. This includes characterising the stability of the AAV vector by monitoring the formation of subvisible particles that can also lead to adverse immunogenic events. Compromised AAV capsid integrity can result in DNA leakage which promotes aggregate formation, potentially lowering product purity and significantly reduce drug efficacy. Understanding how capsid proteins interact with leaked DNA payloads is essential to avoid AAV aggregation and ensure high quality drug product development, control, and shelf-life.
Aura GT’s BMI technology allows high-throughput subvisible imaging, counting and size distribution, and fluorescence applications ID capsids, aggregates and track DNA leakage, to determine AAV stability.
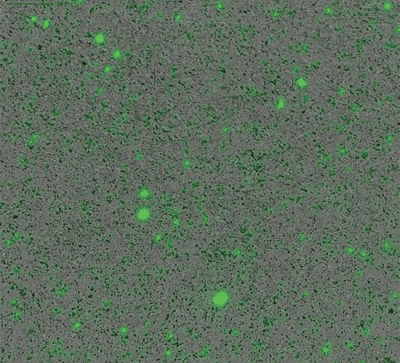
Assess AAV stability
Cell Therapies
Product purity
CAR-T and other cell therapies have advanced personalised medicine to improve cancer patient outcomes. However, cell therapy developers face several challenges when monitoring subvisible particles to ensure product safety and efficacy. Flow cytometry methods are too low-throughput while methods like light obscuration and flow imaging are approximately 60% and 50% accurate, respectively, when cells clump or are out-of-focus. Additionally, distinguishing cells from other SVPs is difficult because cells themselves are subvisible in nature.
Aura CL fluidic-free subvisible particle analysis systems utilise BMI and FMM to ensure accurate count, size, and morphology data and definitively discriminate between cellular, non-cellular, and non-biological contaminants like residual Dynabeads in your cell therapy. Aura’s capabilities are extended with fluorescent detection; determine cell viability, cell type, or perform an immunoassay to distinguish a specific population of cell or protein in a mixed sample.
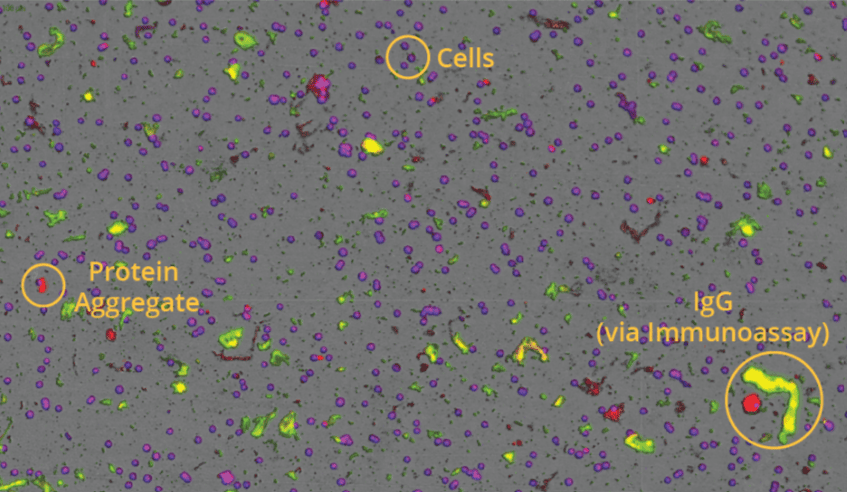
Assess viability and purity in CTs
Small Molecules
An alternative method for solubility measurement
Aqueous solubility of small molecule compounds is an essential parameter in drug discovery and development. Low solubility can impact biological assays, leading to underestimated potency and toxicity, inaccurate SAR, and difficult to interpret ADME results. Despite its critical importance at early stages, other common detection methods for solubility measurement are inefficient, lack sensitivity, or are vulnerable to errors in sample processing.
Aura subvisible particle analysis systems measure compound solubility in high throughput via a simple and flexible workflow. In addition, highly resolved particle images provide valuable information on morphology of precipitated solids for more comprehensive solubility characterisation.

Rapidly assess for solubility

SYSTEMS

Aura PTx
Biologics formulation and development

Aura GT
Ultra-low volume, high-throughput gene therapy quality analysis

Aura CL
Simple, high-throughput cell therapeutic purity analysis

Aura +
One system for all your aggregate and particle analysis needs

Aura
Aggregate and particle counting, sizing and ID

Aura BMI
High-throughput, low volume subvisible particle analysis
| Application | Aura + | Aura CL | Aura GT | Aura PTx | Aura | Aura BMI |
|---|---|---|---|---|---|---|
| Particle Detection / Quantitation | ||||||
| Extrinsic Particles | ||||||
| Protein ID | ||||||
| Polysorbate ID | ||||||
| Cell Aggregate ID | ||||||
| Capsid Aggregate ID | ||||||
| Immunoassays | ||||||
| Cellular Assays | ||||||
| DNA Leakage | ||||||
| High Magnification Microscopy | ||||||
| Custom FL Applications |